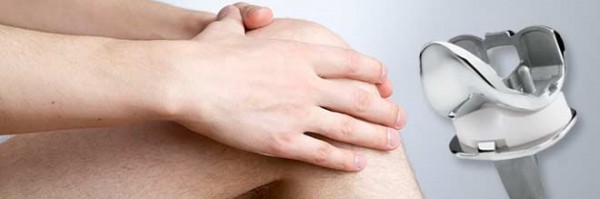
Recently, a married couple filed a lawsuit against Zimmer Orthopedic Surgical Products Inc. They alleged that the company’s knee implant product had defects in design. In addition, they sued Zimmer for negligence and product liability.
Dena Weidenhof and Carl Weidenhof filed the complaint against the defendant (Zimmer Orthopedic Surgical Products, Inc.) on April 13, 2016. They filed their case in the U.S. District Court for the Eastern District of Pennsylvania. The plaintiffs alleged that the company should be held liable for manufacturing a defective knee implant.
The complaint claims that one of the plaintiffs, Carl Weidenhof, suffered injuries that were caused by the implantation of a faulty knee device. To this end, Carl holds the defendants liable for allowing its client to be implanted with a substandard product. According to the plaintiffs, they requested a trial by jury. In addition, they sought for compensatory, exemplary, and punitive damages, interest and court costs, and any relief that could be granted by the courts.
Pogust Braslow & Millrood’s Bharati Sharma and Tobias Millrood as well as Handler, Henning & Rosenberg’s Gregory Feather, David Rosenberg and Mathew Rosenberg represented the plaintiffs.
About Zimmer Manufacturing Company. Founded in 1927, the company designs parts for joint replacement, namely knee and hip products. The company’s knee devices were designed to allow patients enjoy a wider range of motion. However, the company has come under criticism for manufacturing defective knee implants.
These products have been causing increased pain to the victims. In addition, patients have been complaining of premature knee implant failure, expensive revision surgery and loosening of the device.
Besides its knee device, the company has also been on the receiving end about the frequency of injuries caused by the hip products. Zimmer continues to suffer high costs, which emanates from lawsuits related to its faulty products.
Warnings Issued by FDA
The British Medical Journal (BMJ) and the U.S. Food and Drug Administration (FDA) found out that there were numerous problems caused by metal-to-metal devices. According to BMJ’s report, many patients having metal-on-metal products have had higher revision therapy relative to those who have had metal-on-polythene implants.
Most manufacturers of these metal-on-metal components claim that materials such as cobalt, chromium and titanium alloy increase durability. From their reports, the two institutions asserted that use of hip products increases one’s chance of hip dislocation and possibility of contamination of the bloodstream with metallic ions.
Based on rigorous research undertaken by different professional institutions, some patients should not receive metal-on-metal implants. They include people having suppressed immune systems, patients on corticosteroids medication and those that have kidney problems. Others include patients having sensitivity or metal allergy and those that are close to childbearing age.
Zimmer Orthopedic Surgical Products, Inc. Litigation
Most plaintiffs of Zimmer have requested compensation for permanent disability, surgical expenses, pain and suffering, cost of in-home patient care, lost wages and cost of rehabilitation. Some of the doctors have asserted that the company’s implant devices have fundamental design flaws. This is because some knee products have refused to fuse with the thighbones of the patients. As a result, the joints have become loose, thus necessitating revision surgery.
Doctors assert that patients having defective Zimmer products may notice that they have a loose feeling, especially in the replacement knee. Others may have trouble walking or standing. Moreover, some people will hear crunching, popping or clicking noises in the replacement knee. Some patients will have persistent pain and swelling in the replacement knee.
The public has made different claims against Zimmer. Some people have been saying that the company’s marketing tactics deceived them. Most of the lawsuits have accused Zimmer for concealing severe health risks associated with the knee and hip implants.
Mark Sadaka from Sadaka Associates, the leading Defective Product Attorney, has a national practice and works with clients from New York to Alaska.














